A pre-clinical tinnitus drug candidate from Cognosetta has shown promising results (and could soon be ready for human trials), according to new research.
Background:
CS0022 (formerly called BMS-191011) is a next generation BK channel opener designed to lower tinnitus volume by regulating the abnormal neural excitability in the central auditory system (CAS)… thereby restoring the proper “balance of excitation and inhibition” necessary for silence.

The small company behind CS0022 is Cognosetta, Inc. If you have been following our updates here at Tinnitus Treatment Report, that company name might sound familiar.
Just over a year ago, on January 14, 2020, we posted this: Cognosetta, Inc receives funding from NIH to test a novel therapeutic for treating tinnitus…
CS0022-Class Compounds for the Treatment of Tinnitus
Today, we finally have a follow-up about what is happening with that “novel therapeutic” and its development.
First, a quick primer on the company behind the drug. From the Cognosetta website:
Cognosetta is developing a first-in-market, brain targeted pharmaceutical solution for treating tinnitus […] Our priority is developing a first-in-market medication […] for those with moderate to severe tinnitus […] Our scientists have identified a drug target and drug candidates for improving neurophysiological and behavioral measures of tinnitus and age-related hearing loss. To support the clinical success of these drug candidates our scientists are also advancing quantitative methods for evaluating the efficacy of new drug candidates that can be shared across the preclinical and clinical development stages.
Here is a snapshot of their current pipeline, with CS0022 approaching human trials.
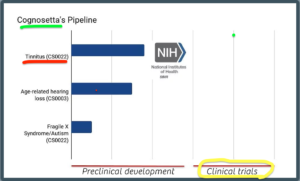
How close to human testing and safety trials is CS0022?
Too early to give an exact date.
But, according to a poster presentation from the upcoming 44th Annual ARO MidWinter Virtual Meeting, the drug continues to impress with promising results in preclinical (animal) studies.
Here is a “sneak preview” copy of the upcoming poster presentation – scheduled for Sunday, February 21, 2021:
The BK Channel Opener CS0022 Mitigates Chronic Tinnitus Following Acoustic Trauma
Background: Subjective tinnitus is an audiological and neurological condition that gives rise to a phantom perception of sound. The condition disproportionately impacts individuals with risky occupational or avocational noise exposure. Patients and animal models of tinnitus exhibit altered central auditory system (CAS) neural activity that is presumed to support the tinnitus percept. Broadly, these changes can be characterized by a dysregulation in the balance of excitation and inhibition that leads to neuronal hyperexcitability and synchrony. The large conductance calcium-activated potassium (BK) channel regulates neural excitability in the CAS and other brain regions. Our recent work suggests that systemic administration of a class of BK channel openers reduces salicylate-induced tinnitus in mice through action in the CAS. The main objective of the current study is to determine whether a similar low-exposure treatment also reduces behavioral and neural manifestations of tinnitus in a chronic tinnitus model induced by noise exposure.
Methods: Auditory brainstem response (ABR) thresholds and acoustic startle behavior were assessed in young adult CBA mice before and after acoustic trauma. For acoustic trauma, awake mice were placed in a small, sound transparent cage, and then exposed for 1 hour via free field to a 113 dB SPL, 1 kHz wide band noise centered at 16 kHz. Ten weeks after damaging noise exposure, mice began systemic treatment with a BK channel opener, CS0022. Gap-prepulse inhibition of the acoustic startle reflex (GPIAS) tracked the development of tinnitus and subsequent effects of treatment. A novel machine learning algorithm and computational analysis method was used to determine which mice displayed tinnitus from the GPIAS protocol.
Results: The acoustic trauma procedure caused limited permanent ABR threshold shifts, ranging from none to moderate (~50 dB) in the high frequencies above 16 kHz. Mice were selected for preliminary analysis if they exhibited minimal permanent hearing loss (< 10 dB) and GPIAS evidence of tinnitus at 16 or 32 kHz after acoustic trauma. Six out of the six selected mice showed less suppressed pre-pulse inhibition for the selected tinnitus frequencies following systemic treatment with a BK channel opener. In contrast, treatment did not influence acoustic startle amplitude functions. Pharmacokinetics studies confirmed low exposure in brain. As expected, improved startle selection via machine learning-enhanced computational analysis increased the power and accuracy for identifying tinnitus and treatment effects.
Conclusions: Ongoing neurophysiological and alternate behavioral measures continue to probe treatment outcomes in this model of acoustic trauma-induced chronic tinnitus. Thus far, preliminary outcomes support the idea that treatment with BK channel openers such as the one used in the current study may reduce evidence of tinnitus. There is currently no effective cure for chronic tinnitus, increasing the urgency for studies exploring the utility of promising drug candidates.
Credit to lead author Luisa Scott of Cognosetta Inc and her co-authors at the University of South Florida.
Thanks to their hard work, it looks like we now have another viable first-in-market tinnitus drug candidate to follow…
Plus, a shiny new meme to help spread the word:

How to get CS0022 updates?
If you want to keep up with promising new tinnitus treatments that are currently in development (including CS0022)… I encourage you to sign up for Tinnitus Treatment Report email updates.
It’s an email newsletter with fresh links and updates (never junk), and… I believe there is no faster way to consistently stay on top of the very latest tinnitus treatment options and scientific breakthroughs.
(It is 100% free, no spam or advertorials, and your information is kept secure and private. Promise.)
Got questions? comments? requests?
Send an email to michael@urgentresearch.com and introduce yourself, say hello.
References and further reading:
- https://braininitiative.nih.gov/funded-awards/developing-novel-therapeutic-treating-tinnitus
- https://projectreporter.nih.gov/project_info_details.cfm?aid=9907138&icde=48273554
- https://www.cognosetta.com
- https://www.nature.com/articles/srep42433
- https://aro.societyconference.com/conf/#sessions/conf10001