A mysterious update related to Neuromod’s neuromodulation device…
Last Tuesday, Neuromod Devices Ltd. quietly updated the official study records of its two active neuromodulation device clinical trials.
The update changed the anticipated Study Completion date from February 2019 to July 2019 – a five month delay.
A similar update occurred back in late December, too. That update also delayed the anticipated Study Completion date for the neuromod device study.
July 2019 or…
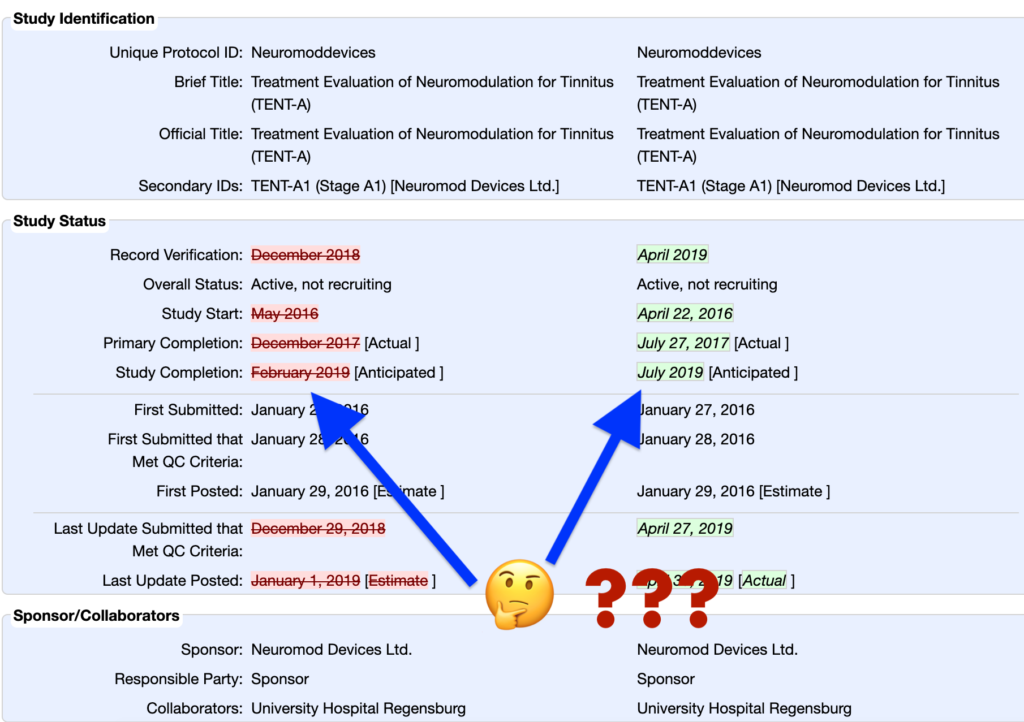
Considering July 2019 is only two months away, this does not appear to be cause for concern.
However, one can’t help but wonder what this means. Without an explanation or comment from Neuromod, this mysterious “delay” could fuel some wild speculation.
Developing…

This post will be updated if and/or when Neuromod provides an update or clarifies the situation.
UPDATE
Dr. Ross O’Neill, CEO of Neuromod commented: “This is a very exciting time for Neuromod as we move towards commercialisation, supported by encouraging data from our recent clinical trials. I am delighted that industry leaders of the calibre of Deb, Suzanne and Cathal have agreed to join our team. Neuromod is investing in growing our organisation; we have been working tirelessly to ensure that all systems are in place to bring our much-anticipated breakthrough treatment to the large population of people living with tinnitus globally.”
SOURCE: https://www.businesswire.com/news/home/20190508005314/en/Neuromod-Broadens-Senior-Leadership-Team-Preparation-Global/
References:
https://clinicaltrials.gov/ct2/show/NCT03530306
https://clinicaltrials.gov/ct2/show/NCT02669069