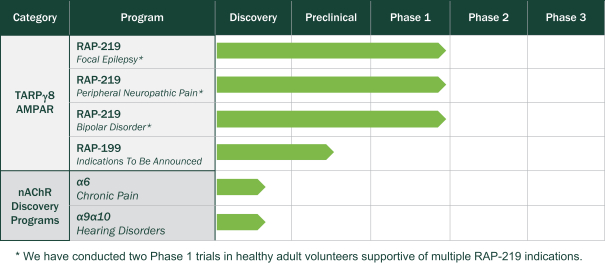
Rapport Therapeutics is a public company that is developing an a9a10 nAChR agonist for the treatment of hearing disorders, including tinnitus.
Information from the company’s SEC filings from June 2024 provide some background and a timeline…
Excerpts from the SEC prospectus, filed June 7, 2024 (before the company went public)
“We are developing an agonist to the a9a10 nAChR for the treatment of hearing disorders, which may include age-related hearing loss, acoustic trauma and tinnitus, as well as vestibular disorders.”
“We are currently developing an oral therapeutic targeting the a9a10 nAChR, which we believe has a high potential target for the treatment of hearing disorders.”
“We believe that a selective agonist of a9a10 nAChR may help treat hearing disorders […]”
“The use of these orally administered molecules in physiological hearing models may demonstrate the potential of a9a10 agonists to address hearing disorders.”

By the way…
Johnson & Johnson Innovation (JJDC) “is the strategic venture capital arm of Johnson & Johnson. JJDC pursues opportunities to solve critical healthcare needs. Our portfolio companies benefit from the full global capabilities of Johnson & Johnson as we collaborate to drive innovation.”
- THIS IS A DRAFT / ROUGH COPY ARTICLE (intended for Tinnitus Treatment Report email subscribers)
From the document:
Our a9a10 nAChR Program
Another program of our interest involves the a9a10 nAChR. We are developing an agonist to the a9a10 nAChR for the treatment of hearing disorders, which may include age-related hearing loss, acoustic trauma and tinnitus, as well as vestibular disorders. Third-party genetic studies in mice have shown that augmenting the a9 nAChR pathway can help prevent hearing loss associated with aging, acoustic trauma and vestibular disorders. Despite this genetic validation, discovery of selective a9a10 nAChR agonists has been challenging because recombinant nAChRs containing a9a10 in cell lines fail to create a functional receptor, as observed with the a6 nAChRs. Our ability to identify agonists that are selective for a9a10 nAChR was made possible by the application of our RAP platform technology. We are currently developing an oral therapeutic targeting the a9a10 nAChR, which we believe has a high potential target for the treatment of hearing disorders. We also believe the a9a10 nAChR is a potential target for the treatment of vestibular disorders, and we may develop an oral product candidate for this indication in the future.
a9a10 nAChR as a Potential Target for the Treatment of Hearing Disorders
In the inner ear, the cochlea converts mechanical sound vibrations into nerve signals, which are transmitted to the brain. Sound vibrations are detected by a combination of outer hair cells, which amplify sound, and inner hair cells (“IHCs”), which receive the amplified sound signals. The IHCs, in turn, translate the incoming signals into release of neurotransmitters, which traverse the synapse to stimulate neurons that send electrochemical signals to the brain. One of the key receptors in this process is the a9a10 nAChR, which is highly enriched in cochlear hair cells.
The role of the a9a10 nAChR in hearing loss has been demonstrated by third-party genetic experiments. Gain and loss of function mutations to the gene encoding a9 demonstrated its role in experimentally induced hearing loss. In these experiments, the thresholds to elicit auditory brain stem responses (“ABR”) to various frequencies of sound were found to be elevated one day after auditory trauma, consistent with hearing loss. In wild-type mice, this effect of auditory trauma was temporary and after seven days, the ABR profile was similar to that observed prior to the insult. In mice with a null mutation of the gene encoding a9, the ABR threshold was increased at day one, and this increase persisted at day seven, demonstrating increased vulnerability to hearing loss. By contrast, mice with a gain-of-function mutation in the gene for a9 were protected from any significant change in ABR on either day one or day seven.
We believe that a selective agonist of a9a10 nAChR may help treat hearing disorders while avoiding many of the side effects that have limited the clinical application of other nAChR modulators.
Preclinical Validation of Our Approach
In vitro studies of a9a10 nAChR physiology have been challenging because this receptor could not be functionally expressed in recombinant cell lines in the absence of it RAPs. Through a genome-wide screen using our discovery platform, RAPs that drive the assembly of functional a9a10 nAChRs were identified by Janssen. Expression of these RAPs along with the a9 and a10 subunits enabled functional a9a10 nAChR expression in cell lines that we believe are suitable for drug discovery.
Janssen conducted a high throughput screen of cells engineered to express a9a10 nAChR and identified a number of small molecule agonists of a9a10. Through our medicinal chemistry efforts, a9a10 agonists with low nanomolar potency, inner ear penetration and high selectivity against other nAChR family members have been identified and are being optimized. The use of these orally administered molecules in physiological hearing models may demonstrate the potential of a9a10 agonists to address hearing disorders.
Rapport Therapeutics background and origins… Johnson & Johnson Innovation and Janssen connections…
We are pursuing agonists and positive allosteric modulators (“PAMs”) of the a6 nAChR for the treatment of chronic pain. Gain-of-function variants in the gene encoding the a6 subunit is responsible for attenuated pain levels. A previous third-party investigational pan-nAChR agonist demonstrated clinical activity in a randomized placebo controlled study in painful diabetic neuropathy but this experimental therapeutic was associated with intolerable side effects that led to the discontinuation of its development. We believe that these side effects were primarily due to the non-selective nature of that agonist. Through our ability to functionally express and pharmacologically screen for a6 nAChR modulators, we have identified small molecule agonists and PAMs that showed a6 nAChR selectivity as well as beneficial activity in a preclinical model of neuropathic pain. We are optimizing these molecules in anticipation of selecting candidates to advance into the clinic.
Our a9a10 nAChR program focuses on the discovery of small molecule modulators of this receptor as potential therapies for hearing disorders. Third-party studies observed a loss-of-function mutation of the gene for the a9 subunit in mice associated with increased sensitivity to noise-induced hearing loss. Conversely, we observed a gain-in-function mutation in a9 protected against hearing loss. We have identified small molecule modulators of a9a10 nAChR and are now optimizing these molecules in anticipation of selecting candidates to advance into the clinic.
Our Company’s History and Our Team
Rapport was formed in February 2022, with founding support from Third Rock Ventures and Johnson & Johnson Innovation-JJDC, to advance the discovery and development of RAP-targeted precision neuromedicines. Our scientific founder and Chief Scientific Officer, David Bredt, M.D., Ph.D., pioneered the discovery of RAPs and their targeting by small molecules while serving as Global Head of Neuroscience Discovery at Janssen Pharmaceutica NV (“Janssen”) and prior to that as Vice President of Neuroscience at Eli Lilly and Company and as a Professor of Physiology at the University of California, San Francisco. Dr. Bredt was subsequently joined at Rapport by additional scientists who previously worked on the RAP platform at Janssen.
Email updates
For updates on the development of a9a10 agonists for tinnitus, and upcoming milestones, sign up for the Tinnitus Treatment Report email newsletter.
References
https://www.rapportrx.com/pipeline/
https://www.sec.gov/Archives/edgar/data/2012593/000119312524157358/d803738d424b4.htm