Updated pipeline graphic on Gateway Biotechnology website reveals three new treatments in preclinical development—including a tinnitus gene therapy and a nasal spray that is advancing toward human trials.
Developing story…
No press release, no announcement, no social media post. But an updated pipeline graphic on the official company website gives us some codenames and just enough context to piece together a bigger picture of what is happening at Gateway right now.
First, the source. A date check on the image points to a June 2023 creation date. Whether it was uploaded at the beginning or end of June, I cannot confirm.
Here is a snapshot of the old pipeline followed by the new pipeline that replaced it.
The old (pre-June 2023) pipeline…
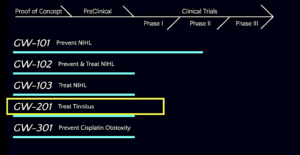
The new and expanded pipeline (latest version)…
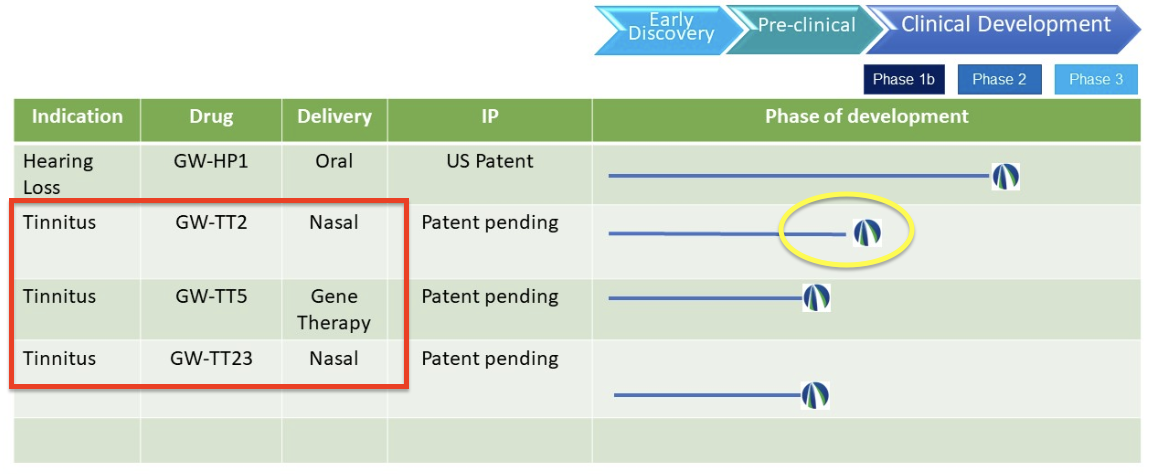
Upon further investigation, there was a brief mention of these new treatments on the company’s LinkedIn page (not dated): “We have also initiated pre-clinical trials to test the efficacy of three drug candidates to treat tinnitus.”
So, we’re looking at two nasal sprays and a gene therapy. One of the nasal sprays is on the edge of pre-clinical is GW-TT2 (circled in yellow in the image of the new pipeline above) and appears to be just shy of a phase 1 clinical trial.
More information about the human trial plans and strategy was hiding nearby on the Gateway Biotechnology website. I say “hiding” because…
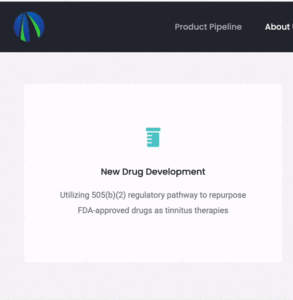
… the website uses an unconventional hover-and-revolve animation that makes the information not immediately apparent.

GW-TT2 (nasal spray)
As for the info about GW-TT2, we’ve retrieved it for your convenience:
“Utilizing 505(b)(2) regulatory pathway to repurpose FDA-approved drugs as tinnitus therapies Recently received positive feedback from FDA in preIND meeting for product development strategy.”
The GW-TT2 nasal spray is apparently an already-approved drug that is being repurposed as a tinnitus therapy. This may speed up both the initiation of clinical trials and eventual approval by months—or even years.
To summarize the little that has been shared about GW-TT2:
- Tinnitus therapy in a nasal spray format
- Advancing toward human trials
- Positive FDA feedback in pre-IND meeting
- Accelerated timeline via 505(b)(2) pathway
GW-TT23 (nasal spray)
GW-TT23 is another nasal spray in the works by Gateway Biotechnology.
- Even less info is available
- Is it a continuation? Combination? Enhancement?
- Code name ambiguous; may be related to GW-TT2 or a typo or, far less likely, a “23rd” drug candidate that followed 16 unchosen candidates
GW-TT5 (gene therapy)
- GW-TT5 — Tinnitus Gene Therapy Targets cells in the auditory pathway Promising pre-clinical animal studies
“Targeted Delivery Utilizing Adeno-Associated Virus (AAV)-based tools to target cells in the auditory pathway (or auditory system) as a tinnitus therapy Promising pre-clinical animal studies demonstrate that we are able to manipulate the tinnitus symptoms with gene therapy”
Jianxin Bao (NEOMED tinnitus researcher)
Meanwhile, CEO Jianxin Bao (aka the NEOMED tinnitus researcher who got millions in funding plus a lot of media coverage two plus years ago) has taken his talents to Duke University, where he’ll be “dedicated to unraveling the mysteries of hearing loss and tinnitus.”
That’s a word-for-word quote from the September 1, 2023 Duke article welcoming Bao to the Department of Head and Neck Surgery & Communication Sciences.
Highlights from that same article:
“Dr. Bao’s research zeroes in on a key cellular process called “synaptopathy,” where the connections between our inner ear’s sensory cells and the brain’s sound-transmitting neurons are lost.”
“Currently, there are no effective medications for these hearing-related issues. But Dr. Bao’s efforts aim to change that. By delving deep into the intricacies of hearing disorders, his team is striving to develop new treatments and preventative measures.”
The warm welcome and enthusiastic language in this article suggests the university is aligned with Bao’s work and the direction of his research. Not everyone gets a welcome post like this, either! You can read the rest here: https://headnecksurgery.duke.edu/news/welcome-jianxin-bao-phd-neuroscientist-researching-hearing-loss-and-tinnitus
Even more exciting, perhaps—also from the Duke website (the careers section)—comes this post: https://careers.duke.edu/job/Durham-POSTDOCTORAL-ASSOCIATE-NC-27710/1009542900/
It’s a job post dated September 18, 2023, but it was first posted about five months ago, in early May.
Here are some interesting excerpts from the job posting:
“The main project for this position is to investigate the neural pathways and molecular pathways involved in noise-induced tinnitus.”
- “Molecular Analysis of Tinnitus”
- “a wide spectrum of approaches”
- “advanced molecular methods … state-of-the-art imaging”
- “sophisticated gene delivery methods”
- “to study cellular and molecular cascades in tinnitus”
“Lead research projects to identify neuronal pathways and molecular cascades for hearing loss and tinnitus based on single cell analysis methods.”
Things are happening.
Lastly, as we wrap up this “Duke is strongly aligned with advanced tinnitus research” theme…
Know who else is at Duke?
Debara Tucci… that’s right, the NIDCD (National Institute on Deafness and Other Communication Disorders) Director!
NIDCD is responsible for funding dozens of projects totaling tens of millions of dollars, related to hearing disorders including tinnitus.
This is a developing story…
Article in progress. More news expected October 23, 2023.
How to get email updates on GW-TT2, GW-TT5, GW-TT23
If you want updates related to these three new treatments in development from Gateway Biotechnology — including the status of human clinical trials — sign up for the Tinnitus Treatment Report email newsletter.
It’s free, your information is kept private, and you can unsubscribe at any time with a single click. Best of all, as a subscriber, you get early access to exclusive updates like this one — before they are added to the front page of this website.
Expect between 1-2 emails per week… but only if something interesting and treatment-related is happening.
References
https://www.gatewaybiotechnology.com/services/
https://www.linkedin.com/company/gateway-biotech/
https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/small-business-and-industry-assistance-frequently-asked-questions-pre-investigational-new-drug-ind
https://www.neomed.edu/news/neomed-researcher-awarded-2-18-million-nih-grant-for-first-human-tinnitus-treatment/
https://headnecksurgery.duke.edu/news/welcome-jianxin-bao-phd-neuroscientist-researching-hearing-loss-and-tinnitus
https://www.nidcd.nih.gov/about/staff/debara-tucci